Photo credit: seminarlinkCs.blogspot.com
Neurohackers dream of the direct neural plug-in, the ultimate hardware-software interface. Ray Kurzweil, scientist/futurist/inventor, has been promoting the transhuman movement for quite some time and recently suggested that nanobots will connect our brains to computers via the cloud by the 2030’s. He envisions the extension into nonbiological thinking as the next step in human evolution. David Eagleman gave a particularly entertaining TED talk on the topic. Besides, it is too late to stop now after William Gibson firmly inserted the notion of a Brain/Computer Interface (BCI) into the cultural narrative with his cyberpunk novel Neuromancer (1984). He coined the term “matrix” and introduced “jacking in”, both used in The Matrix Trilogy whose enormous success made the BCI idea so ubiquitous that its eventuality is assured. I think neurohackers will be pleased with what neuroscience is working on now.
Where are we on the road to the BCI? Is this to be the next phase in human evolution? Interesting questions to ask if you dare to imagine the neuroscience of the future. In fact, there have been amazing advances in the technologies needed to realize a brain/computer interface, and we will look at some of them here, reminded that this is not neurohacking but serious neuroscience conducted by researchers with serious intent.
Two ways to engineer a BCI are on the horizon. The first is to physically interface a computer with neurons using microscopic arrays of electrodes surgically implanted into brain. The second, more interesting, less invasive and more powerful approach is to genetically engineer neurons so that they can be activated or inhibited using noninvasive stimuli such as light, magnetic pulses or sound. We will look at both approaches.
Electrical implants
Implants crafted to stimulate specific brain regions with patterns of electric current have been with us a long time. They are used with good success to treat Parkinson’s Disease symptoms in some patients, or to stimulate the vagus nerve to relieve depression or pain in others. Nowadays, individuals can control a prosthetic limb with their own thoughts using implants and it seems likely that the military seeks to control complex machines in a similar fashion. This is a beginning and one cannot help but wonder what is coming. Modern retinal implants can restore a low level of vision to people blinded by genetic disease, but what will the augmented vision of the future be like? Will we be using implants to enhance attention, alertness or specific skills? Small in-brain computers that give us superhuman capabilities seem like the stuff of sci fi, but the parts are falling into place for a cyborg future.
The art is improving quickly with innovation in materials and implantable microcomputers. But as good as it is, implants are unlikely to deliver the fully functional BCI. They are too invasive, need maintenance and suffer from significant problems, not the least are imprecise positioning and bio rejection. So, what is the path to BCIs that can deliver the functionality that futurists dream of?
TMS – Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is used to study cognition and perception. In TMS a focused external magnetic field delivers pulses to influence nerve cells deep in the brain. It is noninvasive and simply involves holding a magnetic coil near the skull outside the head and passing electric current through the coil. The principle is electromagnetic induction. The magnetic pulse generates an electric current that either activates or inhibits neurons near a focal point depending on the orientation of the field. In this simple form, TMS is imprecise because the path the electric current takes is unpredictable. That is a big limitation because a useful BCI has to provide very well targeted control over neural circuits. So how do we achieve the necessary precision? A combination of genetics and nanotechnology can provide it. Read on.
Stimulation of neurons enabled by cell-targeted nanoparticles
Rapid communication in the neurocircuits of the brain involves the generation of action potentials in neurons and rapid communication between neurons at synapses. These events are well understood. It follows that the BCI must have the ability to precisely stimulate or inhibit action potentials and synaptic transmission in a spatially well targeted way. Roughly speaking the time resolution needs to be finer than +/- 1 millisecond and the spatial resolution finer than +/- 1 micron.
Two groups report success using nanoparticles to stimulate neurons with the appropriate resolution. Carvalho-de-Souza et al. used gold nanoparticles combined with light pulses to activated neurons noninvasively. They bound nanogold to a specific protein that resides in the membrane of a specific subset of neurons. Binding of the gold-conjugate brings the gold particle very close to the nerve membrane. Light pulses delivered by a red laser are absorbed by the gold particles causing them to heat up and the local heating activates the neuron. Because of exquisite molecular targeting, very little heat is required. This clever approach works well with neurons in tissue culture, but there is a lot to do before it can be used in a living brain, plus limited light penetration through the skull is an issue. People are hotly pursuing this and we will see how far it takes us toward the eventual BCI.
A second research group used magnetic nanoparticles to locally activate neurons. Similar to the work with gold, the magnetic particles heat up when exposed to a pulsed magnetic field. In one of their studies cultured neurons that express the ion channel TRPV1 were targeted. The pulsed magnetic field activated the neurons. In a second study, the TRPV1 channel gene was introduced into the ventral tegmental area of mice brain by viral transfection. Magnetic nanoparticles injected into the same area enabled magnetic pulses to activate neurons there and in downstream brain areas. The cool thing with both approaches is that the stimulus, light or magnetic pulse, does not have to be precisely focused because the target specificity is so high. Sound waves will likely work as well, and again could provide a noninvasive stimulus. It is established that light and magnetic pulses can activate neurocircuits , but the whole concept took a huge leap forward with optogenetics, a research tool that is generating tremendous interest.
Optogenetics
Optogenetics is used to study how brains work in laboratory animals. In the future, it may also be important in building a proper BCI. The method uses a combination of techniques derived from optical physics, photochemistry and molecular biology to control and monitor the activity of individual neurons in living animals. Neurons are genetically modified to express light-sensitive ion channels drawn from bacterial genomes. Spatially precise control is achieved because only the cells expressing the gene are activated by light flashes. The method has high spatial and temporal resolution and has been used in animal studies to alter the activity of specific types of neurons in neurocircuits controlling behavior.
The method has tons of potential for research, but to be truly useful for BCI, genetic targeting strategies will have to be improved to deliver light-sensitive actuators with precision to specific populations of neurons in the brain. While that is likely to be an attainable goal, a more difficult problem is the need to deliver light pulses of appropriate wavelength to target neurons deep in the brain. Implanted fiber optic or solid-state light sources are used to do this at present, but this is highly invasive and will be very hard to implement in a human. Then of course, the genes have to be introduced into brain tissue and there are bioethical issues when humans are involved.
Instead of using light, with its limited tissue penetration and strong scattering, people are now looking at magnetic and sound waves as a means of activating neurons genetically modified to be sensitive to those energy sources; sources that pass easily through tissue without the need for surgical intervention.
Transcriptomes & Genome Editing
Both implanted electrodes and noninvasive methods using target molecules provide ways to interface the brain. But to be truly useful it seems inevitable that genomics is going to play a big role in the man-machine interfaces of the future.
Two spectacular developments in molecular biology point the way; transcriptomics and genome editing. We will get to the fundamentals in a moment, but Imagine the possibilities if one could genetically modify specific sets of neurons in a way that they can receive information from computer networks, or even report back to computers, in other words using genetics to enhance consciousness. Hang on to your hat.
Because of huge advances in genome sequencing, it is now possible to identify specific types of neurons by the genes they express, and to do this at the level of single cells extracted directly from known neural circuits. Researchers record the way an individual neuron is connected in a circuit and then extract the RNA from that same cell and sequence it (RNA-seq). Then by comparison against a genome database, they determine the genes the neuron was expressing at the time of the recorded neural activity. This is transcriptomics, and it is not science fiction (see the Neurodata Without Borders project). For example:
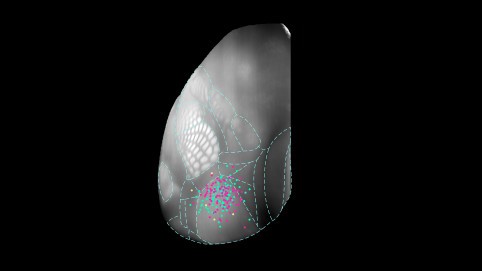 Each of the colored dots in this image is a single neuron detailed in the cell database, clustered in the mouse visual cortex. Allen Institute for Brain Science
Each of the colored dots in this image is a single neuron detailed in the cell database, clustered in the mouse visual cortex. Allen Institute for Brain Science
Technical wizardry to be sure, but here is the kicker- once you know the expressed genes you can use gene editing to place a target gene of your choice into that same class of neurons with absolute precision, a target gene that makes those neurons available to activation under computer control. An example; one might activate a class of neurons to alleviate depression or pain. I think you see how transcriptomics provides the tools needed to enable exquisite, neuron specific targeting for BCI. For discussion of the methods and pitfalls look here. We will take a look at genome editing next
Genome editing with CRISPR/cas9
Genome editing hit the big time with CRISPR/cas9, a molecular tool that allows efficient and precise editing of genes in living cells and organisms. Its introduction is changing molecular medicine, all of biological research and will affect every aspect of our lives. It is not a leap to suggest that gene editing is going to play a huge role in creating the BCI.
Descriptions of the method and discussions of its impact are all over the news and it is something you might like to know about. A good video from MIT describing the method is here. See this too. And we should all celebrate, and take hope in recent results demonstrating the reversal of Duchene Muscular Dystropy by directed gene editing using CRISPR/cas-9 in a mouse model of the disease. Be assured, this is only the beginning.
Cas9 is an enzyme that snips DNA, and CRISPR tells cas9 exactly where to snip. It is a cut-and-paste machine; you can use it to efficiently insert bits of DNA into the genome and you can repair a faulty gene by cutting it out and inserting a normal copy. That’s beautiful and compared to earlier methods, it is easy. You can also position the new gene exactly where you want so that it will be read-out under normal control. What to insert? For our BCI for example, a gene for a protein that makes a neuron sensitive to magnetic pulses. I am quite sure that you can think of lots of applications.
Of course there are ethical questions to consider since people might want to use gene editing to create humans with super strength, appearance, hyper-intelligence, longevity or whatever other genetic traits they desire. For good reason, such possibilities trigger fears of eugenics and designer babies and answers to the big scientific and philosophical questions around CRISPR/Cas9 will certainly fuel lots of discussion. What is clear, however, is that we are hurtling towards a future in which CRISPR/Cas9 will almost certainly have a starring role.
The path to the Cyborg Bio-Bot
Finally, to address our original question, how to make a BioBot, a BCI. On the surface the answer is quite simple, the path will involve detailed knowledge about the connections between neurons, transcriptomics with RNA-seq to uniquely identify neuron sub-types, gene editing with CRISPR/cas9 to introduce activator genes into specific neurons and light, magnetic pulses or sound to stimulate them under computer control. Simple in concept, and quite feasible given enough time for innovation. Remembering that innovation moves quickly, Ray Kurzweil‘s dateline of 2030 might be close to the mark.
The larger question, of course, is should we do it?
-Neuromavin

